- 495
- HENGLU NEWS
On 29th July, the application for authorisation of Lacto-N-triose II (LNT II) as a novel food by Henglu Biotechnology successfully passed the suitability check of the European Food Safety Authority (EFSA), and entered the stage of risk assessment. This milestone progress not only represents a major breakthrough in technological innovation and international market access, but also establishes a steady foundation for the implement of Henglu’s globalization strategy.
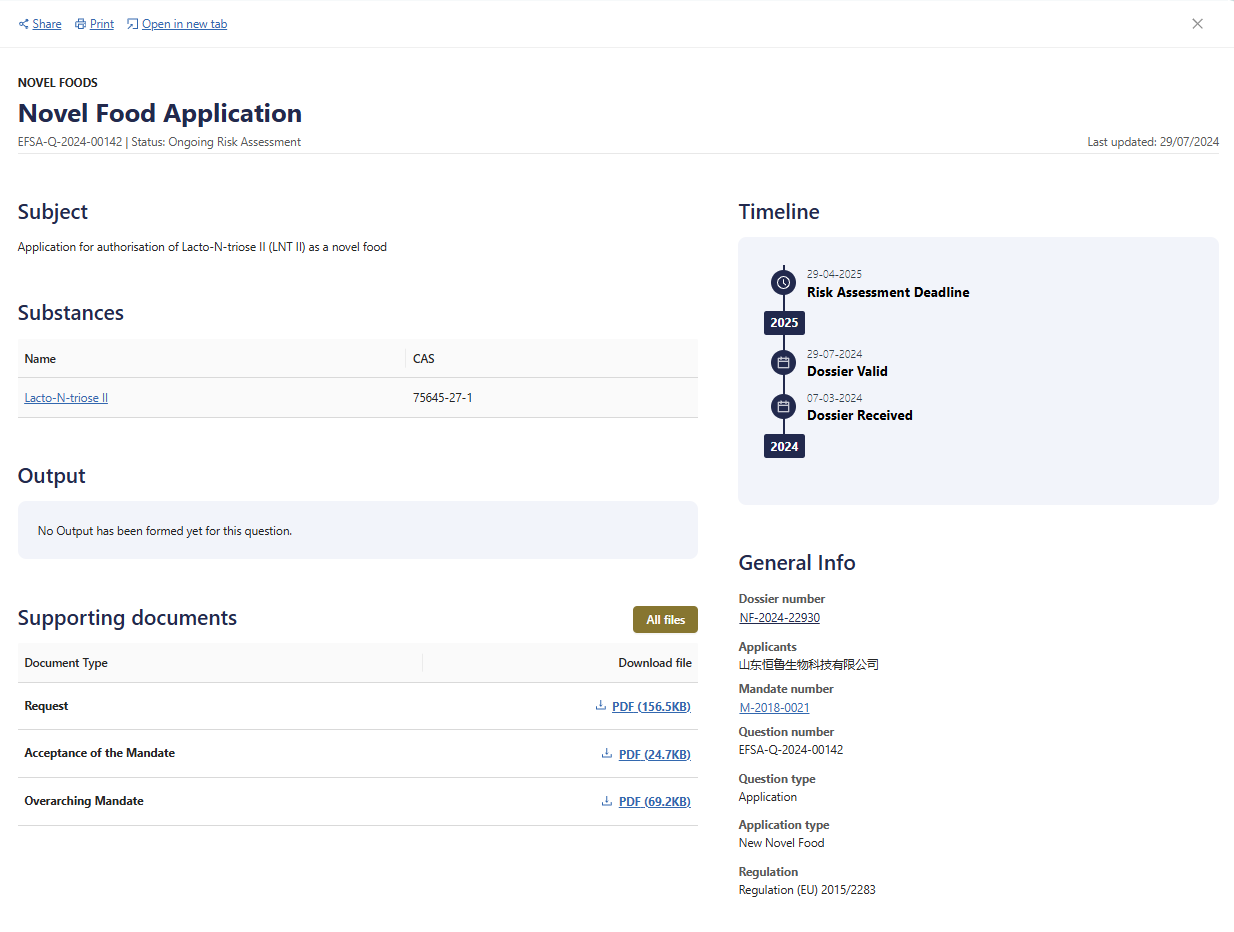
The prequalification for EFSA Novel Food demonstrates the excellent performance and profound accumulation of Henglu’s yeast synthesis technology of human milk oligosaccharides. As the company in the world to use yeast method to achieve mass production of human milk oligosaccharides, Henglu Biotechnology has realized the production of human milk oligosaccharides with innovative technological pathway using lactose/glucose as the carbon source and using yeast to achieve the production of human milk oligosaccharides from scratch. With the company's emphasis on intellectual property work and protection of R&D achievements, Henglu is the forefront in China in terms of the number of patent applications and authorisations on the synthesis of human milk oligosaccharides according to the current patent search results.
In 2022, Henglu Biotechnology made another breakthrough. The LNT II crystalline form was successfully explored and the purity was improved to above 99%. In 2023, the Lacto-N-triose II independently developed by Henglu Biotechnology was even more successful, which not only passed the approval of the National Health Commission for the safety of production strains, but also gained the U.S. FDA Self-GRAS safety certification in the same period. The international recognition has reached a new level.
The series of excellent achievements not only demonstrate the international leading position of Henglu Biotechnology in the field of yeast method technological innovation, but also display the superior ability and forward-looking vision in the approval process and various international certificates.
There is also the opportunity to draw inspiration from the success stories of other companies. For example, in January last year, Ginkgo Bioworks and NAMUH, a US-based infant nutrition company, announced a collaboration to develop human milk oligosaccharides for infant formula. NAMUH’s proprietary technology provides a cost-effective source for human milk oligosaccharides family through yeast fermentation. During this collaboration, NAMUH leveraged Ginkgo’s expertise in yeast strain engineering and fermentation process development to ferment a variety of human milk oligosaccharides via yeast. On the other hand, Amyris has applied the yeast method to a variety of fields such as pharmaceuticals, cosmetics, energy, etc., demonstrating for the industry the unlimited possibilities and wide-ranging value of the yeast method.
The advancement of the yeast method for human milk oligosaccharides production by fermentation is:
Efficient production capacity: High purity human milk oligosaccharides can be produced on a large scale by optimizing fermentation conditions and techniques.
Sustainability: Sustainable production of human milk oligosaccharides using yeast reduces dependence on natural resources.
Cost control: As a common microbial resource and relatively easy to control the culture and fermentation process, yeast can reduce production costs and improve economic efficiency.
Safety: As a fermentation strain, yeast has long been proven to be safe and is less likely to produce harmful substances during production.
As the pioneer in adopting yeast to realize the mass production of human milk oligosaccharides in the international market, Henglu Biotechnology has not only demonstrated its profound technology and forward-looking vision, but also interpreted the core concept of ‘Innovation Leads to the Future’ with practical actions. We are deeply aware of the significant impact of human milk oligosaccharides, as a precious component of breast milk, on the healthy growth of infants and young children. Therefore, Henglu Biotechnology will continue to plough into this field and explore the unknown in order to bring babies around the world nutritional care that is closer to breast milk.



